Abstract
Purpose
Breast cancer susceptibility gene 1/2 can repair damaged DNA through homologous recombination. Besides, the local immune microenvironment of breast cancer is closely linked to the prognosis of patients. But the relationship of breast cancer susceptibility gene 1/2 expression and local immunosuppressive microenvironment in breast cancer is not clear. The aim of this study was to discuss the correlation between them.
Methods
The fresh primary breast tumors and paired normal tissues of 156 cases of breast cancer patients as well as peripheral blood of 156 cases among them in Tianjin Medical University Cancer Institute and Hospital from January 2014 to October 2018 were collected. The association between breast cancer susceptibility gene 1/2 germline mutation and immune status of microenvironment in situ was analyzed.
Results
The results indicated that the germline mutation of breast cancer susceptibility gene 1/2 was inconsistent with the breast cancer susceptibility gene 1/2 protein expression, and the proportion of immune cells in patients with negative expression of breast cancer susceptibility gene 1/2 protein was higher than patients with positive expression of breast cancer susceptibility gene 1/2 protein (p < 0.05). And the expression of programmed cell death protein 1, cytotoxic T-Lymphocyte Antigen 4, programmed death ligand-1 of CD3+ T cells in patients with negative expression of breast cancer susceptibility gene 1/2 protein was higher than patients with positive expression of breast cancer susceptibility gene 1/2 protein (p < 0.05). The breast cancer susceptibility gene 1 protein expression was significantly correlated with family history of breast cancer patients (p = 0.006), local lymph node metastases (p = 0.001), and TNM staging (p ≤ 0.001). The breast cancer susceptibility gene 2 protein expression was significantly related to local lymph node metastases (p ≤ 0.001), III stage rate(p = 0.003) and molecular subtyping (p ≤ 0.001).
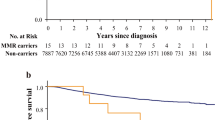
Besides, the 5 years disease free survival was worse for G1 group and pathological III stage patients than other groups and other TNM stage patients.
Conclusion
In short, the immune therapy may be a potential therapy method for breast cancer patients with negative expression of breast cancer susceptibility gene 1/2 protein.








Similar content being viewed by others
Availability of data and material
The datasets used during the current study are available from the corresponding author on reasonable request.
Data transparency
All authors report that all data and materials as well as software application or custom code support our published claims and comply with field standards.
References
DeSantis C, Ma J, Bryan L et al (2014) Breast cancer statistics, 2013. CA Cancer J Clin 64:52–62
Anglian Breast Cancer Study Group (2000) Prevalence and penetrance of BRCA1 and BRCA2 mutations in a population-based series of breast cancer cases. Br J Cancer 83:1301–1308
Balmana J, Diez O, Rubio IT et al (2011) BRCA in breast cancer: ESMO Clinical Practice Guidelines. Ann Oncol Off J Eur Soc Med Oncol 22(6):vi31–vi34
Ford D, Easton DF, Bishop DT et al (1994) Risks of cancer in BRCA1-mutation carriers. Breast cancer linkage consortium. Lancet 343:692–695
Satagopan JM, Offit K, Foulkes W et al (2001) The lifetime risks of breast cancer in Ashkenazi Jewish carriers of BRCA1 and BRCA2 mutations. Cancer Epidemiol Biomarkers Prev 10:467–473
Casey MJ, Bewtra C (2004) Peritoneal carcinoma in women with genetic susceptibility: implications for Jewish populations. Fam Cancer 3:265–281
Robert M, Frenel JS, Gourmelon C et al (2017) Olaparib for the treatment of breast cancer. Expert Opin Investig Drugs 26:751–759
Antoniou A, Pharoah PD, Narod S et al (2003) Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet 72:1117–1130
Chen S, Parmigiani G (2007) Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol Off J Am Soc Clin Oncol 25:1329–1333
Mavaddat N, Peock S, Frost D et al (2013) Cancer risks for BRCA1 and BRCA2 mutation carriers: results from prospective analysis of EMBRACE. J Natl Cancer Inst 105:812–822
Daly MB, Pilarski R, Berry M et al (2017) NCCN guidelines insights: genetic/familial high-risk assessment: breast and ovarian, version 2.2017. J Natl Compr Canc Netw 15:9–20
Geiersbach KB, Samowitz WS (2011) Microsatellite instability and colorectal cancer. Arch Pathol Lab Med 135:1269–1277
Ashworth A, Hudson TJ (2013) Genomics: comparisons across cancers. Nature 502:306–307
Jalkh N, Chouery E, Haidar Z et al (2017) Next-generation sequencing in familial breast cancer patients from Lebanon. BMC Med Genomics 10:8
Kluska A, Balabas A, Paziewska A et al (2015) New recurrent BRCA1/2 mutations in Polish patients with familial breast/ovarian cancer detected by next generation sequencing. BMC Med Genomics 8:19
Lang GT, Shi JX, Hu X et al (2017) The spectrum of BRCA mutations and characteristics of BRCA-associated breast cancers in China: screening of 2,991 patients and 1,043 controls by next-generation sequencing. Int J Cancer 141:129–142
Cropp CS, Nevanlinna HA, Pyrhonen S et al (1994) Evidence for involvement of BRCA1 in sporadic breast carcinomas. Can Res 54:2548–2551
Paul A, Paul S (2014) The breast cancer susceptibility genes (BRCA) in breast and ovarian cancers. Front Biosci (Landmark Ed) 19:605–618
Mirchandani KD, D’Andrea AD (2006) The Fanconi anemia/BRCA pathway: a coordinator of cross-link repair. Exp Cell Res 312:2647–2653
Loi S, Sirtaine N, Piette F et al (2013) Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J Clin Oncol Off J Am Soc Clin Oncol 31:860–867
Fridman WH, Pages F, Sautes-Fridman C et al (2012) The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 12:298–306
Ceilley E, Jagsi R, Goldberg S et al (2005) Radiotherapy for invasive breast cancer in North America and Europe: results of a survey. Int J Radiat Oncol Biol Phys 61:365–373
Recht A, Edge SB, Solin LJ et al (2001) Postmastectomy radiotherapy: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol Off J Am Soc Clin Oncol 19:1539–1569
Bartholomeusz C, Xie X, Pitner MK et al (2015) MEK inhibitor selumetinib (AZD6244; ARRY-142886) prevents lung metastasis in a triple-negative breast cancer xenograft model. Mol Cancer Ther 14:2773–2781
Stevens KN, Vachon CM, Couch FJ (2013) Genetic susceptibility to triple-negative breast cancer. Can Res 73:2025–2030
Basourakos SP, Li L, Aparicio AM et al (2017) Combination platinum-based and DNA damage response-targeting cancer therapy: evolution and future directions. Curr Med Chem 24:1586–1606
Lord CJ, Tutt AN, Ashworth A (2015) Synthetic lethality and cancer therapy: lessons learned from the development of PARP inhibitors. Annu Rev Med 66:455–470
Litton JK, Rugo HS, Ettl J et al (2018) Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med 379:753–763
Lyons TG, Robson ME (2018) Resurrection of PARP inhibitors in breast cancer. J Natl Compr Canc Netw 16:1150–1156
Robson M, Im SA, Senkus E et al (2017) Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med 377:523–533
Tung NM, Garber JE (2018) BRCA1/2 testing: therapeutic implications for breast cancer management. Br J Cancer 119:141–152
Han HS, Dieras V, Robson M et al (2018) Veliparib with temozolomide or carboplatin/paclitaxel versus placebo with carboplatin/paclitaxel in patients with BRCA1/2 locally recurrent/metastatic breast cancer: randomized phase II study. Ann Oncol 29:154–161
Litton JK, Scoggins M, Ramirez DL et al (2017) A feasibility study of neoadjuvant talazoparib for operable breast cancer patients with a germline BRCA mutation demonstrates marked activity. NPJ Breast Cancer 3:49
Loibl S, O’Shaughnessy J, Untch M et al (2018) Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial. Lancet Oncol 19:497–509
Alexandrov LB, Nik-Zainal S, Wedge DC et al (2013) Signatures of mutational processes in human cancer. Nature 500:415–421
Nicola MD, Apetoh L, Bellone M et al (2017) Innovative therapy, monoclonal antibodies and beyond. Cytokine Growth Factor Rev 38:1
Ballas ZK (2018) The 2018 nobel prize in physiology or medicine: an exemplar of bench to bedside in immunology. J Allergy Clin Immunol 142:1752–1753
Wang J, Yuan R, Song W et al (2017) PD-1, PD-L1 (B7–H1) and tumor-site immune modulation therapy: the historical perspective. J Hematol Oncol 10:34
He Y, Rivard CJ, Rozeboom L et al (2016) Lymphocyte-activation gene-3, an important immune checkpoint in cancer. Cancer Sci 107:1193–1197
Woo SR, Turnis ME, Goldberg MV et al (2012) Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res 72:917–927
Ascierto PA, Bono P, Bhatia S et al (2017) Efficacy of BMS-986016, a monoclonal antibody that targets lymphocyte activation gene-3 (LAG-3), in combination with nivolumab in pts with melanoma who progressed during prior anti–PD-1/PD-L1 therapy (mel prior IO) in all-comer and biomarker-enriched populations. Ann Oncol 28:v611
Lipson EJ, Tawbi AH, Schadendorf D et al (2021) Relatlimab (RELA) plus nivolumab (NIVO) versus NIVO in first-line advanced melanoma: primary phase III results from RELATIVITY-047 (CA224-047). J Clin Oncol 39:9503–9503
David S, Hong PS, Aitana C et al (2018) Phase I/II study of LAG525 ± spartalizumab (PDR001) in patients (pts) with advanced malignancies. J Clin Oncol 36:3012–3012
Loi S, Michiels S, Salgado R et al (2014) Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol Off J Eur Soc Med Oncol 25:1544–1550
Menard S, Valagussa P, Pilotti S et al (2001) Response to cyclophosphamide, methotrexate, and fluorouracil in lymph node-positive breast cancer according to HER2 overexpression and other tumor biologic variables. J Clin Oncol Off J Am Soc Clin Oncol 19:329–335
Moul JW (2010) Radiotherapy: secondary malignancies after prostate cancer treatment. Nat Rev Clin Oncol 7:249–250
Hodi FS, O’Day SJ, McDermott DF et al (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723
Narayan P, Wahby S, Gao JJ et al (2020) FDA approval summary: atezolizumab plus paclitaxel protein-bound for the treatment of patients with advanced or metastatic TNBC whose tumors express PD-L1. Clin Cancer Res 26:2284–2289
Barroso-Sousa R, Jain E, Cohen O et al (2020) Prevalence and mutational determinants of high tumor mutation burden in breast cancer. Ann Oncol 31:387–394
Szekely B, Bossuyt V, Li X et al (2018) Immunological differences between primary and metastatic breast cancer. Ann Oncol 29:2232–2239
Nanda R, Liu MC, Yau C et al (2020) Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early-stage breast cancer: an analysis of the ongoing phase 2 adaptively randomized I-SPY2 trial. JAMA Oncol 6:676–684
Schmid P, Cortes J, Pusztai L et al (2020) Pembrolizumab for early triple-negative breast cancer. N Engl J Med 382:810–821
Loibl S, Untch M, Burchardi N et al (2019) A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: clinical results and biomarker analysis of GeparNuevo study. Ann Oncol 30:1279–1288
Baretta Z, Mocellin S, Goldin E et al (2016) Effect of BRCA germline mutations on breast cancer prognosis: a systematic review and meta-analysis. Medicine (Baltimore) 95:e4975
Brianese RC, Nakamura KDM, Almeida F et al (2018) BRCA1 deficiency is a recurrent event in early-onset triple-negative breast cancer: a comprehensive analysis of germline mutations and somatic promoter methylation. Breast Cancer Res Treat 167:803–814
Antoniou AC, Casadei S, Heikkinen T et al (2014) Breast-cancer risk in families with mutations in PALB2. N Engl J Med 371:497–506
Cunningham JM, Cicek MS, Larson NB et al (2014) Clinical characteristics of ovarian cancer classified by BRCA1, BRCA2, and RAD51C status. Sci Rep 4:4026
Moschetta M, George A, Kaye SB et al (2016) BRCA somatic mutations and epigenetic BRCA modifications in serous ovarian cancer. Ann Oncol 27:1449–1455
Sridhar KS, Hussein AM, Feun LG et al (1989) Activity of pirarubicin (4’-0-tetrahydropyranyladriamycin) in malignant mesothelioma. Cancer 63:1084–1091
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12:252–264
Keir ME, Butte MJ, Freeman GJ et al (2008) PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 26:677–704
Liang SC, Latchman YE, Buhlmann JE et al (2003) Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur J Immunol 33:2706–2716
Flies DB, Sandler BJ, Sznol M et al (2011) Blockade of the B7–H1/PD-1 pathway for cancer immunotherapy. Yale J Biol Med 84:409–421
Iwai Y, Ishida M, Tanaka Y et al (2002) Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA 99:12293–12297
Blank C, Gajewski TF, Mackensen A (2005) Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother 54:307–314
Berger R, Rotem-Yehudar R, Slama G et al (2008) Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res Off J Am Assoc Cancer Res 14:3044–3051
Brahmer JR, Drake CG, Wollner I et al (2010) Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol Off J Am Soc Clin Oncol 28:3167–3175
Hamid O, Robert C, Daud A et al (2013) Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 369:134–144
Topalian SL, Hodi FS, Brahmer JR et al (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443–2454
Greenwald RJ, Latchman YE, Sharpe AH (2002) Negative co-receptors on lymphocytes. Curr Opin Immunol 14:391–396
Ward FJ, Dahal LN, Wijesekera SK et al (2013) The soluble isoform of CTLA-4 as a regulator of T-cell responses. Eur J Immunol 43:1274–1285
Topalian SL, Taube JM, Anders RA et al (2016) Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer 16:275–287
Liang B, Workman C, Lee J et al (2008) Regulatory T cells inhibit dendritic cells by lymphocyte activation gene-3 engagement of MHC class II. J Immunol 180:5916–5926
Huard B, Prigent P, Pages F et al (1996) T cell major histocompatibility complex class II molecules down-regulate CD4+ T cell clone responses following LAG-3 binding. Eur J Immunol 26:1180–1186
Pena J, Jones NG, Bousheri S et al (2014) Lymphocyte activation gene-3 expression defines a discrete subset of HIV-specific CD8(+) T cells that is associated with lower viral load. Aids Res Hum Retrov 30:535–541
Burugu S, Gao D, Leung S et al (2017) LAG-3+ tumor infiltrating lymphocytes in breast cancer: clinical correlates and association with PD-1/PD-L1+ tumors. Ann Oncol Off J Eur Soc Med Oncol 28:2977–2984
Schmidt MK, van den Broek AJ, Tollenaar RA et al (2017) Breast cancer survival of BRCA1/BRCA2 mutation carriers in a hospital-based cohort of young women. J Natl Cancer Inst 109:djw329
Cortesi L, Masini C, Cirilli C et al (2010) Favourable ten-year overall survival in a Caucasian population with high probability of hereditary breast cancer. BMC Cancer 10:90
Gonzalez-Angulo AM, Timms KM, Liu S et al (2011) Incidence and outcome of BRCA mutations in unselected patients with triple receptor-negative breast cancer. Clin Cancer Res Off J Am Assoc Cancer Res 17:1082–1089
Kriege M, Seynaeve C, Meijers-Heijboer H et al (2009) Sensitivity to first-line chemotherapy for metastatic breast cancer in BRCA1 and BRCA2 mutation carriers. J Clin Oncol Off J Am Soc Clin Oncol 27:3764–3771
Acknowledgements
We thank Li Dong, Yingnan Ye for technical assistance.
Funding
None.
Author information
Authors and Affiliations
Contributions
JL and JY performed study concept and design; NW and LW development of methodology and writing, review and revision of the paper. FL and LL provided acquisition, analysis and interpretation of data, and statistical analysis.
Corresponding authors
Ethics declarations
Conflict of interest
The authors report no conflicts of interest for this work.
Ethical approval
This research project was approved by the Ethics Committee of Tianjin Cancer Institute and Hospital. Ethics approval for breast tumors, paired normal tissues as well as peripheral blood: use of breast tumors, paired normal tissues as well as peripheral blood was approved by the ethics committee of the Ethics Committee of Tianjin Cancer Institute and Hospital.
Informed consents
Written informed consents were obtained from each patient in accordance with the Declaration of Helsinki. Written consents were obtained from each patient to publish the pathological tissue images as representative figures.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Wu, N., Wei, L., Li, L. et al. Perspectives on the role of breast cancer susceptibility gene in breast cancer. Int J Clin Oncol 27, 495–511 (2022). https://doi.org/10.1007/s10147-021-02098-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-021-02098-1